bromine configuration|Bromine Electron Configuration (Br) with Orbital Diagram : Tagatay Mar 23, 2023 Cada Nota Fiscal Eletrônica ou Recibo Eletrônico de Serviço possui uma Assinatura Digital única que permite verificar sua autenticidade dentro do sistema, para isso, basta clicar em VALIDAR NF-e no menu a esquerda. Além disto, todas as cartas de correção emitidas estarão vinculadas a NF-Eletrônica original.https://www.officialtinytexie.com/wp-content/uploads/2020/03/IMG-0259.jpghttps://www.officialtinytexie.com/wp-content/uploads/2020/03/IMG-0727.jpg
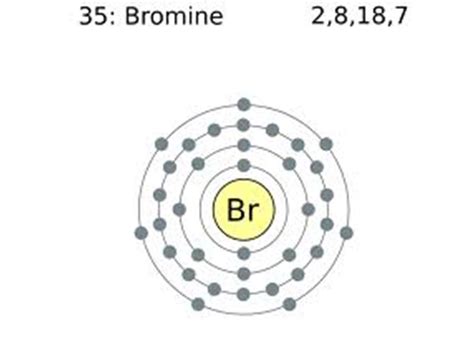
bromine configuration,Mar 23, 2023 Bromine is extracted by electrolysis from natural bromine-rich brine deposits in the USA, Israel and China. It was the first element to be extracted from seawater, but this is now .Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between .Bromine Electron Configuration (Br) with Orbital Diagram The bromine electron configuration, denoted as [ Ar] 4s 2 3d 10 4p 5 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5, showcases the precise placement of electrons within the atom.
Bromine Ground-State Electron Configuration. The complete electron configuration of the Bromine is: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 5. You can also see the image given below;
Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine has .

Bromine electron configuration. ← Electronic configurations of elements. Br (Bromine) is an element with position number 35 in the periodic table. Located in the IV period. Melting .The ground state electronic configuration of neutral bromine is [ Ar ]. 3d10. 4s2. 4p5 and the term symbol of bromine is 2P3/2. Bromine: description. Bromine is the only liquid . Explanation: All you need to do is work your way across the periodic table filling the orbitals as you go. The full version of this is: 1s22s22p63s23p64s23d104p5. .Its from n=3 onwards that its 18 per shell for n=4 its 32 so the configuration for bromine would be 2.8.18.7 with 7 in outer shell as expected. 1 Report. Reply. Reply 4. 13 years ago. ervo. 5. Technically that's true because it's the 3d orbitals that take the extra 10 electrons, but if you look at the periodic table, for GCSE level this would . Introduction. The method of unambiguously assigning the handedness of molecules was originated by three chemists: R.S. Cahn, C. Ingold, and V. Prelog and is also often called the Cahn-Ingold-Prelog .bromine configuration Bromine Electron Configuration (Br) with Orbital Diagram Absolute Configurations of Perspective Formulas. Chemists need a convenient way to distinguish one stereoisomer from another. The Cahn-Ingold-Prelog system is a set of rules that allows us to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ’ (from the Latin rectus, .NOTE: Chromium is an exception to the rules for writing electron configurations! Video: Cr, Cr 2+, and Cr 3+ Electron Configuration Notation In writing the electron configuration for Chromium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chromium go in the 2s orbital.Bromine is a chemical element; . Bromine has the electron configuration [Ar]4s 2 3d 10 4p 5, with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising agent, .Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. . Electron configuration The arrangements of electrons above the last (closed shell) noble gas. Melting point The temperature at which the solid–liquid phase change occurs.In the case of $\ce{Br-}$ it is an anion of charge minus 1 meaning that it will have one more electron than a normal bromine atom. So really its electron configuration will be the same as krypton which is the atom after bromine. So all you have to do is add an extra electron in the last orbital, the 4p orbital. The initial configuration type of zinc–bromine static batteries, which was proposed by Barnartt and Forejt , consisted of two carbon electrodes immersed in a static ZnBr 2 electrolyte and separated by a porous diaphragm. In this design, an activated charcoal layer was pasted on the positive electrode that was vertically oriented in the .
Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of electrons in each orbital, it is just a matter of how the electronic configuration notation is written (here is an explanation why). Therefore we have 1s 2 2s 2 2p 6 3s 2 3p 6 3d .
The noble gas configuration is a shorthand electron configuration for atoms. In chemistry, the noble gas configuration is a shorthand method of writing an atom’s electron configuration.The reason for using the noble gas configuration is because the full electron configuration becomes very long for atoms with high atomic .bromine configurationLa configuration électronique du brome est : 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁵. Le brome, également connu sous le nom de feu liquide, est défini comme un élément chimique faisant partie du tableau périodique des éléments. Determine the electron configuration of bromine, then write it in the appropriate form. Use the appropriate Slater Rule to calculate the shielding constant for the electron. Solution A Br: 1s 2 2s 2 2p 6 3s 2 .
Study with Quizlet and memorize flashcards containing terms like The electron configuration for hydrogen (H), The electron configuration for bromine (Br), The electron configuration for aluminum (Al) and more. The correct electron configuration of bromine(Br) in the ground state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p x 2 4p y 2 4p z 1. Valency and valence electrons of bromine. This electron configuration shows that the last shell of a bromine atom has an unpaired electron. So in this case, the valency of bromine is 1.E/Z and Cis/Trans Alkenes. In the previous post, we talked about the cis and trans designation of alkenes. As a recap, cis and trans stereoisomerism depends on whether two identical alkyl groups on the c=c bond are on the same or opposite sides of that double bond: The cis and trans approach works only if two identical groups are connected to the .
The configuration at the left hand double bond is E; at the right hand double bond it is Z. Thus this compound is (1E,4Z)-1,5-dichloro-1,4-hexadiene. The double-bond rule in determining priorities. Example 4. Consider the compound below:
Bromine electron configuration diagram. On the basis of the following diagram, the electronic configuration diagram of Br can be explained. The maximum number of electrons that can fit into these subshells, S, P, D, and F, respectively, are 2, 6, 10, and 14, and they fill up in increasing order of energy.
The electron configuration shows the distribution of electrons into subshells. This list of electron configurations of elements contains all the elements in increasing order of atomic number.. To save room, the configurations are in noble gas shorthand.This means part of the electron configuration has been replaced with the .
bromine configuration|Bromine Electron Configuration (Br) with Orbital Diagram
PH0 · What is the electron configuration for bromine?
PH1 · WebElements Periodic Table » Bromine » the essentials
PH2 · Electron configuration for Bromine (element 35). Orbital diagram
PH3 · Electron Configuration Chart of All Elements (Full Chart)
PH4 · Complete Electron Configuration for Bromine (Br, Br
PH5 · Bromine electron configuration
PH6 · Bromine Electron Configuration (Br) with Orbital Diagram
PH7 · Bromine (Br)
PH8 · Bromine